Our Comprehensive Research Solutions

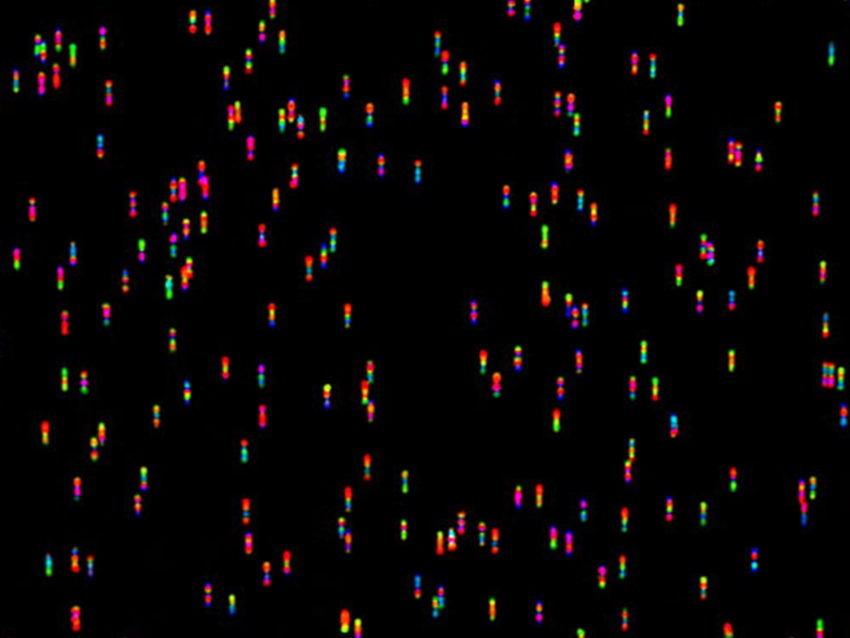
nCounter® Analysis System
Rapidly translate scientific discoveries into actionable gene expression insights with 800-plex direct digital detection.
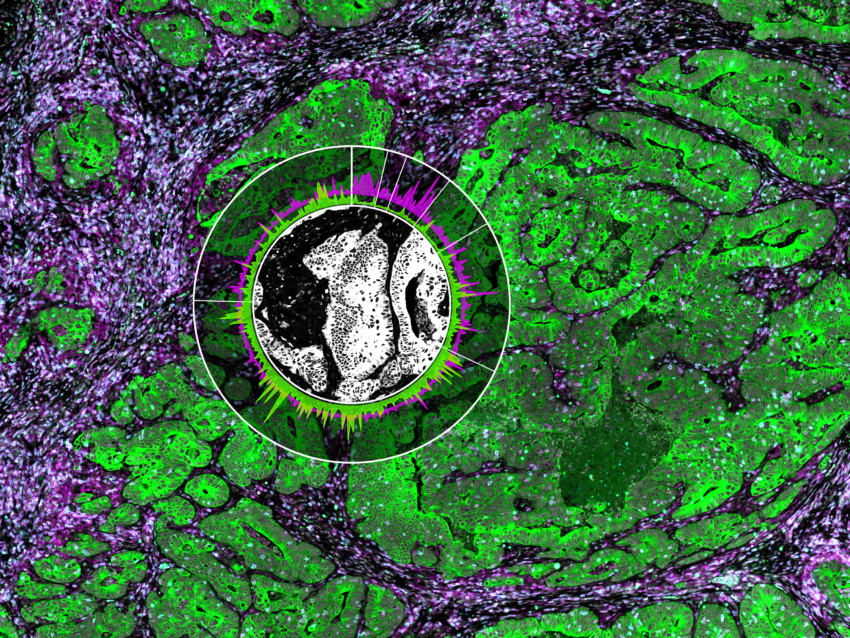
GeoMx® Digital Spatial Profiler
Spatially profile the whole transcriptome and 150+ protein targets from FFPE and fresh frozen tissue.
CosMx™ Spatial Molecular Imager
High-plex, multiomic, spatial single-cell imaging that delivers deeper insights for cell atlasing, cellular interactions, and biomarker discovery.
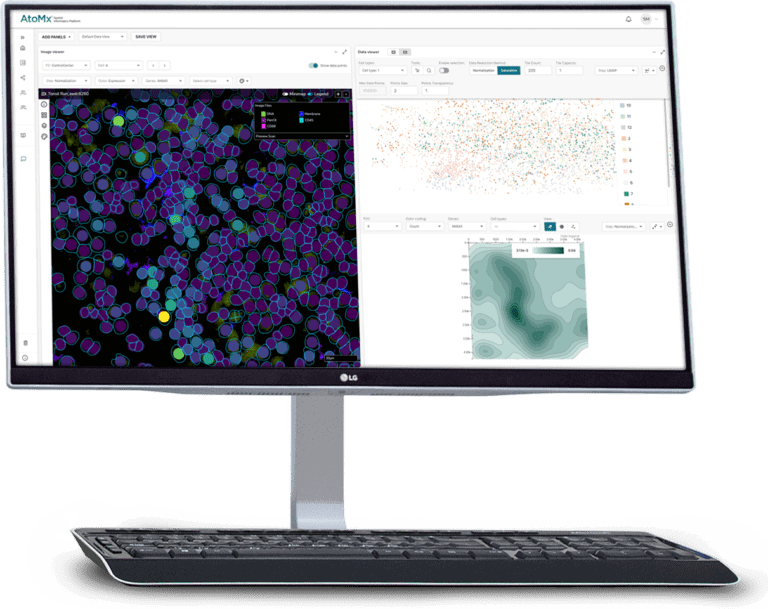
AtoMx™ Spatial Informatics Platform
Leverage the cloud for spatial data analysis and visualization to obtain meaningful insights from spatial multiomics data.
The CosMx™ SMI and decoder probes are not offered and/or delivered to the Federal Republic of Germany for use in the Federal Republic of Germany for the detection of cellular RNA, messenger RNA, microRNA, ribosomal RNA and any combinations thereof in a method used in fluorescence in situ hybridization for detecting a plurality of analytes in a sample without the consent of the President and Fellows of Harvard College (Harvard Corporation) as owner of the German part of EP 2 794 928 B1. The use for the detection of cellular RNA, messenger RNA, microRNA, ribosomal RNA and any combinations thereof is prohibited without the consent of the President and Fellows of Harvard College (Harvard Corporation).

Make the Leap to Single-Cell Spatial
CosMx™ Human 6K Discovery Panel
Shipping Now
Unlock Unprecedented Biology with 6000 Genes
Improving the
Human Condition
“The CosMx SMI instrument has become integral to our laboratory’s future direction, and the responsiveness and skill of the customer support for it and the AtoMx SIP has been absolutely fantastic.”

Dr. Grant Kolar
Director, Research Microscopy and Histology Core, St. Louis University School of Medicine
“The CosMx SMI complemented our efforts to generate organ atlases of the healthy human system together with our partners of the Human Cell Atlas project. It allowed us to visualize single-cell reference maps directly in organs. The scale of 1000-plex gene panel with image-guided cell segmentation makes the CosMx SMI, a unique platform for spatial genomics!”

Dr. Holger Heyn
Team Leader, Single Cell Genomics Group, CNAG, Spain
“We are impressed with ease of use with which the CosMx SMI enabled single cell spatial transcriptomics across various tissue types, even in archival FFPE tissue. The high-plex protein assay makes the CosMx SMI a true multiomic solution. The CosMx SMI’s large capture area allows analysis from valuable clinical trial samples using tissue microarrays, enabling characterization of 100s of samples weekly.”

Dr. Nigel B Jamieson
Professor of Surgery, University of Glasgow, UK
“The NanoString cancer panels, in general, and the Breast Cancer 360 Panel, in particular, in combination with the outstanding analytical tools developed by NanoString, provide straightforward, informatically-rich tools for characterization of the functional genomic landscape of breast cancer.”

E. Aubrey Thompson, PhD
Professor of Cancer Biology, Mayo Clinic
“There is a big movement underway to study the microenvironments of cancers in different organs because it is known that immunotherapy doesn’t work the same across different cancer types. GeoMx will be the key to us to understand those mechanisms. It will be able to see things we only dreamed about—it will be revolutionary”

Nina Radosevic, MD
Surgical & Molecular Pathologist / Cell Biologist, Associate Research Professor
University Clermont Auvergne, INSERM, Centre Jean Perrin
“We plan on utilizing the nCounter PanCancer IO 360 Gene Expression Panel as we move on to Digital Spatial Profiling with the GeoMx system to generate some of our gene signatures and explore new regions of interest.”

Troy McEachron, PhD
Assistant Professor of Research, University of Southern California
“The deciding factor in obtaining the GeoMx DSP was its ability to look at both transcriptomic and proteomic information.”

Peter O’Toole, PhD
Director of the Bioscience Technology Facility, University of York
“One of the great things about NanoString is that it can be quickly adapted for all sorts of applications.”

Edward Weinstein, PhD
CEO, Canopy Biosciences
“nCounter allowed us to use flash-frozen paraffin embedded tissues that were a few years old, as well as didn’t have necessarily the best quality RNA, and still get really great data from it.”

Emily Schwarz
Ohio State University
“The GeoMx DSP is a workhorse in the lab for hypothesis testing… Complementing the single-neuron resolution of GeoMx, we rely on the CosMx SMI to achieve true subcellular spatial analyses and uncover cell-cell interactions… NanoString’s consistent advancements in panel development and plexity provide us with the confidence that they will continue empowering neuroscientists to explore complex questions.”

Miranda Orr, PhD
Assistant Professor, Gerontology and Geriatric Medicine, Wake Forest School of Medicine

Catalyzing understanding
7,000 Publications and counting
Browse the vast and growing catalog of peer-reviewed research from oncology to biomarker discovery enabled by NanoString technologies.
Careers
Become a Part of the Spatial Biology Revolution
“I have really enjoyed becoming a part of the company and teaching our customers more about the technologies NanoString offers and how to apply them to push their research forward.”

“NanoString is a uniquely innovative place to work, as we get to do far more exciting and cutting-edge science than most companies our size. We experience the thrill of building technologies alongside the satisfaction that comes from participating in research advancing patient care for a broad range of disease areas.”

“At NanoString Technologies I have a great opportunity to work with outstanding engineers and be part of the leading edge of gene expression instrumentation.”





Spotlight